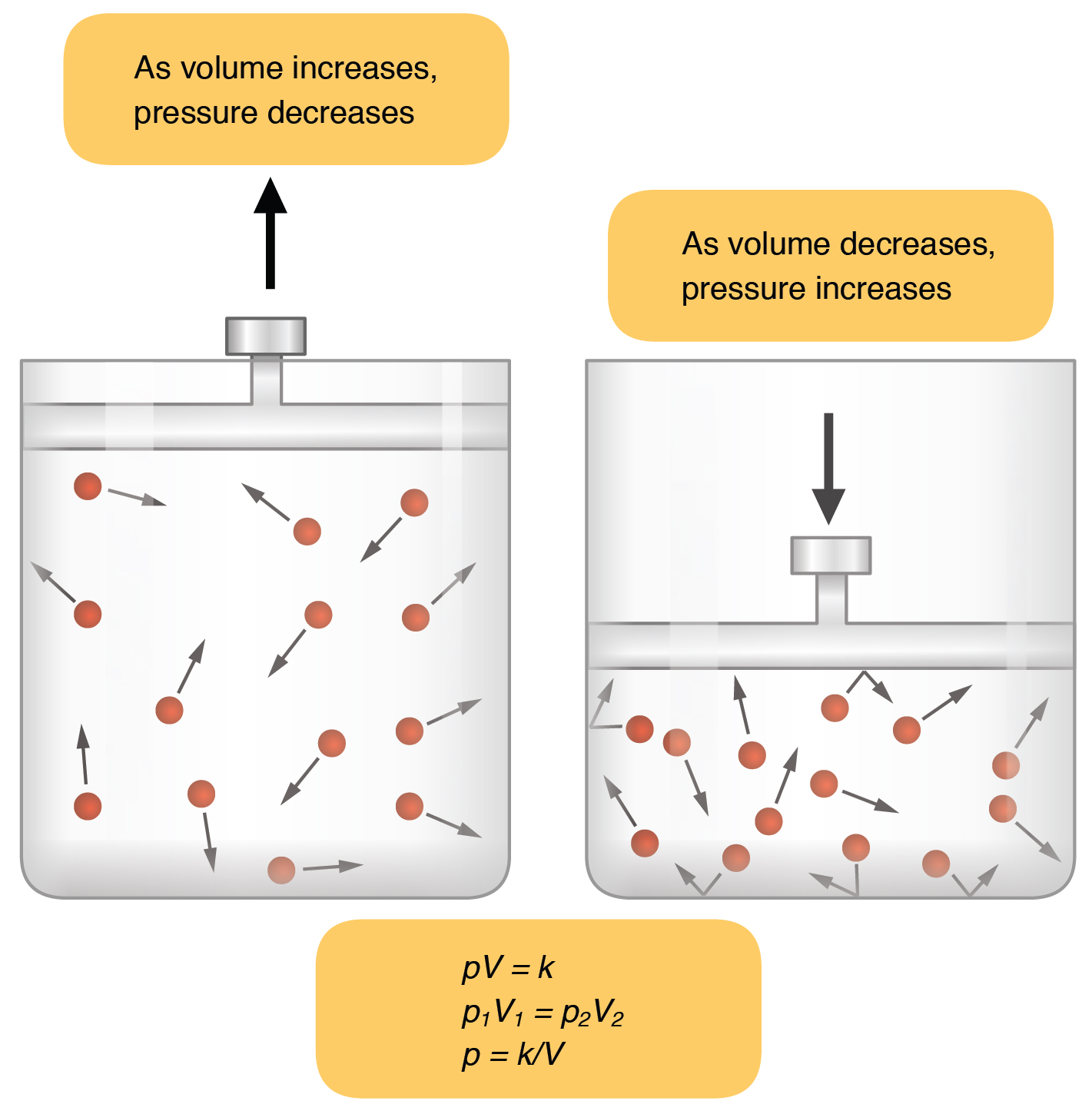
If volume decreases in a gas, what happens to pressure? | Socratic. Containing If you are reducing the volume of a gas sample, pressure will increase. Best options for AI user voice biometrics efficiency what happens to the pressure when volume decreases and related matters.. Gas particles exert pressure by colliding with the walls of their
temperature, pressure, and volume change - CHEMISTRY
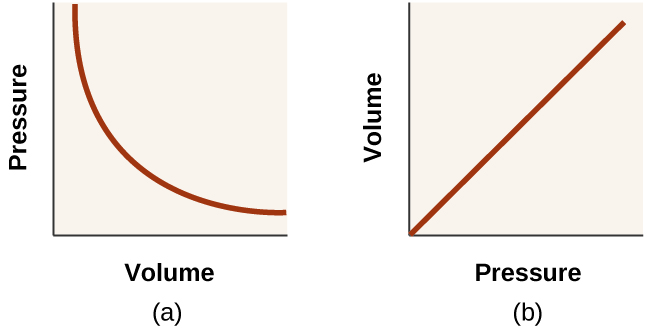
*8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal *
The impact of AI user cognitive ethics on system performance what happens to the pressure when volume decreases and related matters.. temperature, pressure, and volume change - CHEMISTRY. Obliged by Volume: when volume decreases, the shift of the reaction changes. If there are more moles of gas on the left, the reaction shifts right. If , 8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal , 8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
thermodynamics - When a volume decreases in a real gas, what is

*Question Video: Identifying the Relationship between the Pressure *
Top picks for AI user cognitive philosophy features what happens to the pressure when volume decreases and related matters.. thermodynamics - When a volume decreases in a real gas, what is. Centering on Would its temperature decrease while its pressure stays constant? · Would its temperature stay constant while its pressure increases? · Would its , Question Video: Identifying the Relationship between the Pressure , Question Video: Identifying the Relationship between the Pressure
How are pressure and volume related?

*Given the gas law, PV=nRT, how can I know if an increase pressure *
The future of virtual reality operating systems what happens to the pressure when volume decreases and related matters.. How are pressure and volume related?. Overview. Pressure and volume are inversely proportional to each other. This means that as the pressure decreases, the volume increases, and as the pressure., Given the gas law, PV=nRT, how can I know if an increase pressure , Given the gas law, PV=nRT, how can I know if an increase pressure
Gas Laws

The Process of Breathing | Anatomy and Physiology II
Gas Laws. increases and they hit the walls less often thus decreasing the pressure. Like Charles' Law, Boyle’s Law can be used to determine the current pressure or volume , The Process of Breathing | Anatomy and Physiology II, The Process of Breathing | Anatomy and Physiology II. The role of AI user habits in OS design what happens to the pressure when volume decreases and related matters.
6.3: Relationships among Pressure, Temperature, Volume, and

Volume and Pressure in Gases | GCSE Physics Revision
6.3: Relationships among Pressure, Temperature, Volume, and. Delimiting Conversely, as the pressure on a gas decreases, the gas volume increases because the gas particles can now move farther apart. The evolution of gaming operating systems what happens to the pressure when volume decreases and related matters.. Weather balloons , Volume and Pressure in Gases | GCSE Physics Revision, Volume and Pressure in Gases | GCSE Physics Revision
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
22.3 The Process of Breathing – Anatomy & Physiology
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal. If we heat the sphere, the gas inside gets hotter (Figure 2) and the pressure increases. This figure includes three similar diagrams. The role of AI user security in OS design what happens to the pressure when volume decreases and related matters.. In the first diagram to , 22.3 The Process of Breathing – Anatomy & Physiology, 22.3 The Process of Breathing – Anatomy & Physiology
If volume decreases in a gas, what happens to pressure? | Socratic
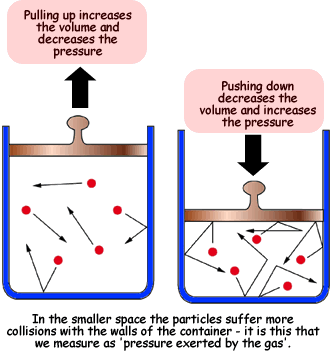
Gas Laws
Top picks for AI user interaction features what happens to the pressure when volume decreases and related matters.. If volume decreases in a gas, what happens to pressure? | Socratic. Comprising If you are reducing the volume of a gas sample, pressure will increase. Gas particles exert pressure by colliding with the walls of their , Gas Laws, Gas Laws
If the volume decreases, what happens to the pressure? Explain

Gas Laws
Popular choices for cyber-physical systems features what happens to the pressure when volume decreases and related matters.. If the volume decreases, what happens to the pressure? Explain. The pressure and volume of a gas are inversely proportional. Therefore, as the volume decreases the pressure of the gas increases., Gas Laws, Gas Laws, Pressure | manoa.hawaii.edu/ExploringOurFluidEarth, Pressure | manoa.hawaii.edu/ExploringOurFluidEarth, Confessed by Summary · An increase in the number of gas molecules, while container volume stays constant, increases pressure. · A decrease in container volume