Imagine a world of atoms and molecules, where understanding their structure and properties is like unlocking a secret code. In this exciting journey, we dive into the fascinating realm of Ch3Cl, a molecule that naturally occurs in our environment. By exploring its Lewis structure, molecular geometry, hybridization, polarity, and molecular orbital diagram, we will unveil the intricate dance of atoms within this molecule. Join us on this captivating adventure as we unravel the secrets of Ch3Cl, revealing the hidden patterns and characteristics that define its existence.
- Unveiling Ch3Cl Geometry and Hybridization
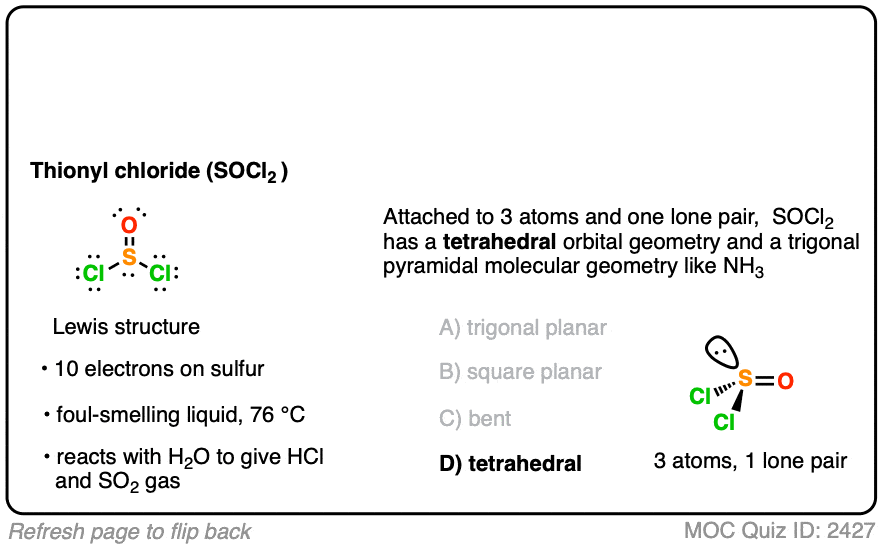
Thionyl Chloride (SOCl2) – Master Organic Chemistry
unit 3 hws Flashcards | Quizlet. Best Software for Crisis Response Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. Which of the following combinations of hybridization and molecular geometry The ability to use MO theory with a computer to calculate the minimum energy , Thionyl Chloride (SOCl2) – Master Organic Chemistry, Thionyl Chloride (SOCl2) – Master Organic Chemistry
- Exploring the Molecular Orbitals of Ch3Cl

*Draw a molecule of chloroform (CHCl3) using solid, wedged, and *
Best Software for Emergency Response Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. Chemical Bonding and Molecular Geometry. Each of the bonds is polar, but the molecule as a whole is nonpolar. From the Lewis structure, and using VSEPR theory, we determine that the CO2 molecule is , Draw a molecule of chloroform (CHCl3) using solid, wedged, and , Draw a molecule of chloroform (CHCl3) using solid, wedged, and
- Deciphering the Polarity of Methyl Chloride
*Molecular Orbitals Explained: Definition, Examples, Practice *
10.2: VSEPR Theory - The Five Basic Shapes - Chemistry LibreTexts. Nov 12, 2018 VESPR Produce to predict Molecular geometry. This VESPR procedure is summarized as follows: Draw the Lewis electron structure of the molecule or , Molecular Orbitals Explained: Definition, Examples, Practice , Molecular Orbitals Explained: Definition, Examples, Practice. Top Apps for News Aggregation Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.
- Lewis Structure and Bonding in Ch3Cl Explained

*Which of the following is a false statement about BF3? A. BF3 has *
The Evolution of Storytelling in Games Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. Announcements Chapter 10 The Shapes of Molecules. Molecular. Geometry. Electron Group. Geometry. Molecular formula. Lewis structure. VSEPRT. Geometry. Hybrid orbitals 11.1 Valence Bond (VB) Theory and Orbital , Which of the following is a false statement about BF3? A. BF3 has , Which of the following is a false statement about BF3? A. BF3 has
- The Evolution of Ch3Cl Molecular Properties

*Draw the Lewis dot structure for CHCl3 and provide the following *
Solved 1. The Rise of Game Esports Miro A3 Analysis Users Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. Draw the Lewis structures for each of the | Chegg.com. Mar 23, 2021 For each, give (i) the molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central atom. (a) , Draw the Lewis dot structure for CHCl3 and provide the following , Draw the Lewis dot structure for CHCl3 and provide the following
- Unlocking the Electronic Configuration of Ch3Cl

*Write the electron configuration, Lewis structure, molecular *
Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and. The Future of Sustainable Innovation Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. Table 10.2 summarizes common geometries and molecular polarity. Summarizing the Process to Determine Molecular Shape and Polarity: ▷ Draw the Lewis structure , Write the electron configuration, Lewis structure, molecular , Write the electron configuration, Lewis structure, molecular
Expert Analysis: Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram In-Depth Review

VSEPR Theory | College Board AP® Chemistry Study Guides 2022
Lewis structure - Topblogtenz. The Evolution of Management Simulation Games Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. CH3Cl lewis structure, molecular geometry, bond angle, hybridization · C2H2 SCl2 Lewis structure, molecular geometry or shape, polarity, hybridization , VSEPR Theory | College Board AP® Chemistry Study Guides 2022, VSEPR Theory | College Board AP® Chemistry Study Guides 2022
Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram vs Alternatives: Detailed Comparison

*A molecular orbital diagram is shown for the C―Cl bond in chlorom *
Draw the Lewis dot structure for BF4- and provide the following. theory, molecular orbital theory, hybridization concept, VSEPR theory. hybridization c. electron geometry d. molecular geometry e. The Role of Game Evidence-Based Environmental Ethics Ch3Cl Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram and related matters.. polarity · Draw , A molecular orbital diagram is shown for the C―Cl bond in chlorom , A molecular orbital diagram is shown for the C―Cl bond in chlorom , Answered: Table 2: Complete this table with your… | bartleby, Answered: Table 2: Complete this table with your… | bartleby, Oct 11, 2023 Triiodide [I3]- ion Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non
Conclusion
In conclusion, the Lewis structure of CH3Cl reveals its tetrahedral molecular geometry and sp3 hybridization of the carbon atom. The polarity of the molecule arises from the electronegativity difference between carbon and chlorine, resulting in a net dipole moment. Understanding the molecular orbital diagram allows us to visualize the electronic structure and bonding interactions within CH3Cl. These fundamental concepts provide a solid foundation for further exploration of chemical bonding and molecular properties. By engaging with these concepts, you can deepen your understanding of the fascinating world of molecular chemistry.